
In response to concerns from some colleagues regarding possible interpretations of my comments about an article by a Cleveland Clinic group in the Newsletter of the Brazilian Society of Tropical Medicine the last January 15, I will add further clarification.
For now, the commented article is still in the pre-print stage, however many high-impact journals require articles submitted to the journal to be published in one of the public archives in the pre-print format. Likely, the article is not yet in its final version, but the main data or conclusions will hardly be significantly modified and soon the article will be published in some journal after the article is properly reviewed by other experts. (https://www.medrxiv.org/content/10.1101/2022.12.17.22283625v1.full.pdf)
The article warns of the occurrence of a well-known phenomenon in immunology since the late 1950s. Dr. Thomas Francis Jr, a pioneer in identifying this phenomenon, used it in the title of his article “On the Doctrine Of Original Antigenic Sin” (ref Proceedings of the American Philosophical Society, Dec. 15, 1960, Vol. 104, No. 6 (Dec. 15, 1960) pp. 572-578), therefore crystallizing the name of this phenomenon as term Original Antigenic sin (OAS). This phenomenon has been described very clearly in their studies with influenza A virus.
The history the fact within influenza is well presented in a more recent paper (The Journal of Infectious Diseases 2017;215:1782–8). Briefly, what it means is that the first infection by one type of influenza A interferes with the immune responses against other types of influenza A and a new name, “negative interference” that more explicitly defines the phenomenon of OAS was proposed. The possibility of OAS in Covid-19 vaccines was widely discussed at the beginning of development and their potential was assessed by regulatory agencies. The fact that there were no significantly different variants that generated concern in the first year of the pandemic caused the topic to be quickly forgotten.
However, with the emergence of immunologically distinct Omicron variants, vaccine manufacturers have developed a new formulation of the vaccine to also cover the Omicron BA.1 variant antigen and the issue of OAS was again brought to light as a topic of interest to immunologists and public health managers. In an immunological study of bivalent vaccine-induced immune responses published in Science Immunology (Kaku et al., Sci. Immunol. 7, eabq3511 (2022), the authors demonstrate that after immunization with the original vaccine, or prior infection with the Omicron variant, immune responses are predominantly against antigens that are common to the two variants and develop very little specific response against Omicron. Another study with non-human primates (Gagne et al., 2022, Cell 185, 1556) also demonstrates that the predominant response is against the common antigens demonstrating a negative interference of the previous immunity.
These aspects of the vaccine and OAS phenomenon are commented in an editorial in The New England Journal of Medicine published on January 11, 2023 written by Paul A. Offit (Offit PA. Bivalent Covid-19 Vaccines – A Cautionary Tale. N Engl J Med. 2023 Jan 11. doi: 10.1056/NEJMp2215780. Epub ahead of print. PMID: 36630616). In addition to this, a more detailed analysis of the immune mechanisms involved in Covid-19 OAS was published in a review in the Journal of Clinical Investigation (Clin Invest. 2023;133(1):e162192. https://doi.org/10.1172/JCI162192).
In the article from the investigators of the Cleveland Clinic, the authors followed immunizations and symptomatic infections of Covid-19 in a large cohort of health professionals and found a low (30%) effectiveness of the bivalent vaccine protecting symptomatic infections from Omicron BA.4 and BA.5 variants and other more recent variants. The authors then, investigated the factors associated with low vaccine effectiveness. First, they investigated the impact of previous infections by strains that existed before Omicron and Omicron variants on the protective effectiveness against variants BA.4 and BA.5 and the more recent BQ.1, BQ.1.1, and BF.7 variants. The article’s figure 1 shows the impact of previous infections on the presentation of symptomatic Covid-19 disease. And it shows that infections with pre-delta and delta variants offer very little protection compared to people who have never been infected while infections with Omicron variants BA1 and BA2 offer moderate protection and those already infected with BA4 and BA5 did not present reinfections.
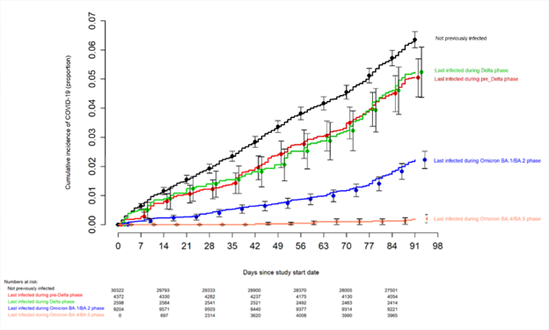
Figure. Simon-Makuch plot comparing the cumulative incidence of COVID-19 for subjects stratified by the number of COVID-19 vaccine doses previously received. Day zero was 12 September 2022, the day the bivalent vaccine began to be offered to employees. Point estimates and 95% confidence intervals are jittered along the x-axis to improve visibility.
On the article´s figure 2 the authors investigate the correlation between the number of doses of the original virus vaccine and the effectiveness of the bivalent vaccine in protecting against BA4 and BA5 infections. The graph shows the cumulative incidence of symptomatic cases of Omicron variant infections in people who received zero to more than three doses and a clear inverse correlation is observed between the number of doses of the original monovalent vaccine and the level of protection against symptomatic Omicron infections.
This does not mean that the original vaccine is not protective. The original vaccine was very effective against all variants up to the delta variants and still is very effective against those variants, but, due to viral adaptation, the virus found ways (new variants) to escape the immunity of the population.
The possibility of increasing antibody-dependent infection has been discussed since the beginning of the pandemic as several studies have demonstrated the phenomenon of ADE in other coronaviruses that infect animals. Indeed, there are several publications on ADE in coronaviruses in general (Wen J, Cheng Y, Ling R, Dai Y, Huang B, Huang W, Zhang S, Jiang Y. Antibody-dependent enhancement of coronavirus. Int J Infect Dis. 2020 Nov;100:483-489. doi: 10.1016/j.ijid.2020.09.015. Epub 2020 Sep 11. PMID: 32920233; PMCID: PMC7483033). ADE in COVID-19 has already been proposed (Lee WS, Wheatley AK, Kent SJ, DeKosky BJ. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat Microbiol. 2020 Oct;5(10):1185-1191. doi: 10.1038/s41564-020-00789-5. Epub 2020 Sep 9. PMID: 32908214.) by several researchers since the beginning of the pandemic. However, to date there is no conclusive evidence to indicate that the severity of the disease or the increased transmission rate is caused by ADE, while some experiments and several published cases demonstrate that this effect is possible.
However, until now, the other variants were not as immunologically divergent as the Omicron, to be taken as a different serotype, because neutralizing antibodies against the original virus do not neutralize the new variants. Faced with clear evidence of OAS in Covid-19, which is one of the requirements for ADE, there is even more concern about the possibility that ADE can occur as a mechanism for the survival of the SARS-CoV-2 in the population. There are several forms of ADE. The most well-known happens in dengue, which is mediated by existing heterotypic pre-infection antibodies while other forms of ADE are mediated by antibodies induced during infection and by homotypic antibodies at low concentrations.
However, the example of dengue ADE does not mean the same will happen with Covid-19. The fact that the findings of the article of effectiveness of the bivalent vaccine are not what we would like does not mean that we should ignore them, nor does it mean that immunization has been deleterious to the population in any way; thanks to this vaccine, variants Alpha, Gamma and Delta, which are much more pathogenic than Omicron, had their circulation in the population suppressed. In addition to neutralizing antibodies, other protective mechanisms are induced by Covid-19 vaccines, such as complement opsonization and cellular responses, and which can be improved by including non-structural Covid-19 proteins or other forms. That is why I propose to monitor more closely the signs of ADE emergence in clinical presentations of the disease as well as to promote more sophisticated Covid-19 vaccine developments that can protect a wider spectrum of SARS-CoV-2 variants.
Finally, I declare that I do not know the authors of the Cleveland Clinic, nor do I have projects to develop any type of Covid-19 vaccine, nor do I participate in committees that recommend vaccine purchases.










