
ReBEC: local platform, global science
Vanguard in Brazil and in the world, the virtual platform is the only primary registry in the world in Portuguese recognized by the WHO
08/12/2021
Aimed at researchers, health professionals and other interested parties, the ReBEC concentrates information from studies involving the recruitment of human beings for testing new drugs and clinical procedures
To be an open-access virtual platform to meet national and regional information needs and provide transparency to experimental and non-experimental clinical trials carried out on human beings by Brazilian and foreign researchers, in progress or completed, in Brazilian territory. This is the mission of the Brazilian Registry of Clinical Trials (ReBEC), one of the 16 primary registries in the world accredited by the World Health Organization (WHO) through its global network, the International Clinical Trials Research Platform (ICTRP Network) and the only one in Portuguese to also assimilate the mandatory guidelines of the International Committee of Medical Journal Editors (ICMJE). Accreditations guarantee the Brazilian registry the same acceptance, by the most prestigious journals globally, such as Lancet and Nature, as the other members of the ICTRP network and Clinical Trials, with which ReBEC maintains direct collaboration for research carried out in Brazil since 2017 and holds the management of studies at the Oswaldo Cruz Foundation (Fiocruz) at the American base since 2014.
Dr. Luiza Silva, coordinator of ReBEC, explains that it is a platform for recording studies carried out in Brazil, even when part of an international multicentric initiative. Also, according to her, any prospective study that assigns human participants, or groups of human beings, to one or more health-related interventions whose effects on people will be evaluated must be registered in the ReBEC. Clinical trials have been described lately as the gold standard in the evaluation of issues related to treatment and prevention in health. For no other reason, the registry of clinical trials, initially proposed with the aim of respecting ethical reasons involving recruitment, is today an important source of evidence on the effectiveness and reliability of health interventions, provided that it is carried out on specific platforms, the primary registries, duly accredited”, he points out.
Such interventions aim to modify a health outcome and may include drugs, surgical procedures, devices, behavioral treatments, among others. The constitution of a database on clinical research in Brazil in a single site gathers information that is often dispersed in different institutions, which were not necessarily published or were always accessible to interested parties. When comparing ReBEC with Clinical Trials and other databases, José Luiz dos Santos Filho, reviewer of clinical trials at ReBEC, points out a series of advantages. “For all audiences here in the country, be they study participants, researchers or other interested parties, the biggest advantage, without a doubt, is the personalized service in Portuguese, followed by the search system and the intuitive interface,” he points out. For registrants, the facility to start and update a registry and the manual to help fill in the required information.
Santos Filho emphasizes that in ReBEC it is possible to register observational and retrospective studies (those in which the clinical trial begins before registration on the platform), although these are not accepted by all journals. As it is publicly owned, open access and free of charge for anyone who wants to register or consult approved records, it is also a tool for advocacy groups and social control. “The advantage for the general public is the transparency of the platforms statistics, available at the bottom of the website, and the availability of the full content in Portuguese, always,” he highlights.
The platforms coordinator also claims that ReBEC increases the effectiveness of clinical trials efforts by making the studies widely known, reducing the always feared publication bias. For her, the transparency of the registry can increase the effectiveness in clinical trials and current observational studies for at least two reasons. “The first is that, as researchers planning new studies become aware of existing trials, unnecessary duplication of research efforts, including costs, can be reduced, and cooperation between experts and institutions can be fostered. The other is that free access to registered studies that are recruiting participants can increase the attraction rates of these individuals, especially for clinical trials on rare diseases or those involving high-risk conditions, details Dr. Silva.
Dr Josué Laguardia, ReBECs senior consultant on clinical research, notes that one of the results of these combined processes would be an increase in the chances of successful outcomes of some trials, which contributes, along with several other factors, to add value to the results research and improve evidence to guide health practices and policies. “This is because it is a reliable, curated, internationally accredited and unbiased source of information. This credibility is what guarantees recognition by excellent journals and consistency of information for systematic reviews, meta-analyses and evidence-based guidelines,” he says.
A history of innovation and readiness
This month, ReBEC completes 11 years of continuous operation. When it was first launched, it was a pioneer initiative of its kind in the country and the only one in the world with 100% open code. In less than a year it became one of the primary ICTRP registries. At the end of the initial allocation for the platforms implementation, in 2014, constant changes in the Ministry of Health made the projects sustainability difficult. “The solution was via research and technological development projects. After that, the Ministry made a complementary contribution that allowed us to finance improvements to the platform, develop an application for recruitment (still unpublished), attend to health emergencies and pay staff, for a while,” says the coordinator. The result came quickly: there are fast-tracks in place since 2016 for express approval within 48 hours of studies with correct documentation on Zika, dengue, yellow fever, and malaria.
The Covid-19 pandemic would be, as it was for everyone, the biggest challenge. “We signaled to Fiocruzs presidency the need for a fast-track even in January 2020, without imagining that Brazil would go through a real nightmare. I used the latest resources to buy cell phones for the reviewers and, when the lockdown arrived, we were ready to migrate from telephone service and email routines, provided for in regulation, to an on-call system from 8 am to 20 pm, with direct interaction: continuously, via chat, or even by appointment, by cell phone”, recalls Dr. Silva.
Also according to her, the change was a success. “We greatly accelerated the speed of approvals for the registrations as a whole, by reducing doubts directly with the registrants,” recognizes the coordinator. As for the fast-track for Covid, it made Brazil a highlight in the number of studies registered on the WHO platform. Paradoxically, with the pandemics priorities here in the country, there were even more delays in personnel payments and team dismissals; it took a while, but we managed to get a dedicated budget in October this year,” he celebrates.
International leading role
Despite the advances, the ReBEC was on the verge of losing its accreditation in the ICTRP and recognition by the ICMJE, as the system developed with the front-end, back-end and compliance updates had not gone live due to bureaucratic problems. A direct contact between the coordination and the Committees summit reversed the situation, scoring a goal for the Brazilian platform: the ReBEC donated the platforms code to the WHO and maintained the tradition of the worlds only primary registry with 100% open code, now with a state-of-the-art alternative to primary registries for the international developer community.
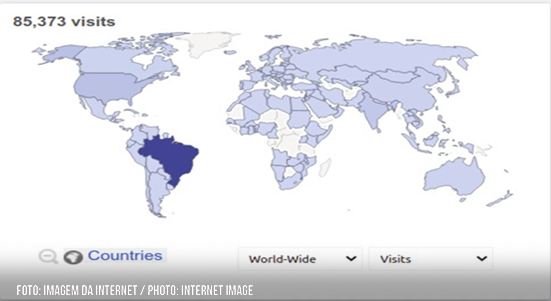
Global accesses to ReBEC in 2021. Source: Matomo Analytics
The platform has considerable visibility outside the country: until November 18 in 2021, it had more than 57 thousand accesses from Brazil and 28 thousand from other countries – a third of the total. “The primary bilingual registry allows for a gain in efficiency that boosts knowledge and cooperation between specialists and research groups, there is no doubt about it,” guarantees the coordinator. As the records are also published in English as required by the WHO, where ReBEC replicates its base on a monthly basis, it not only gives global visibility and transparency to the information on clinical research carried out in Brazil, whether by pharmaceutical industries, Brazilian or foreign researchers, but also increases the potential for reuse of scientific and technological information in health by both the international and regional community.
“We have an important role for Portuguese-speaking researchers, specifically, around the world. But now we want to increase the regional protagonism. Spanish is available on the system, but its use is still very small,” he acknowledges. “In size, we are second only to Clinical Trials in the Americas. By collaborating to meet the demand currently being faced by Cuba and Peru, the only members of the ICTRP in Spanish, we will be able, together, to build a virtuous alliance of transparency for all studies carried out in the countries of Latin America and the Caribbean. It is not a dream, it is a global health agenda,” bets Dr. Silva.
Understand the process and the numbers
Launched in December 2010, the platform reaches the end of 2021 with an all-new system and more than five thousand study records, 3,000 of these recruiting. Asked whether the ReBEC has any mechanism to guarantee the validity of the registered data, Dr. Laguardia clarifies that the studies submitted are validated in four stages: one is the registration with the ethics committee of the research institution that proposes the trial, the so-called Consubstantiated Opinion (CEP), or the opinion of the National Council for Research Ethics (Conep). Another is the Certificate of Presentation of Ethical Appreciation (CAAE), issued by Plataforma Brasil (national and unified database of research records involving human beings for the entire system, CEP/Conep) as soon as the study is approved by the committees or by Conep. “The registration stage at ReBEC is also a validation, since submission on the platform is subject to the presentation of opinions issued by the research ethics committees of the research institutions themselves or by the national committee,” he adds. Finally, the studies approved by ReBEC are migrated to the base of the International Clinical Trials Registry Platform (ICTRP), which is the global registry managed by WHO.
On November 17, the platform had a total of 11,037 submitted clinical trials, but this does not exactly represent a sum of the other statistics on the platform, as some of these approved trials request a new review, or the recruiting study may have already been approved, for example. In total, 5,181 studies have been approved, all with validation accepted by the most important scientific journals in the world and open to public consultation, 4,102 clinical trials are being drafted and 208 are being analyzed.
“As the ReBEC is a platform for the submission of clinical trials run in Brazil, most of the studies are Brazilian, whether they are self-funded or publicly funded, through state research support foundations or the National Council for Scientific and Technological Development (CNPq) and the Coordination for the Improvement of Higher Education Personnel (Capes), elucidates Santos Filho. It is a joint project of the Ministry of Health, the Institute of Scientific and Technological Communication and Information in Health (Icict/Fiocruz), the Pan American Health Organization (PAHO) and the Latin American and Caribbean Center for Science Information da Saúde, also known by its original name Regional Medicine Library (BIREME). Its executive committee is composed of the aforementioned institutions and the National Health Surveillance Agency (Anvisa).










