
Case report: Implantable cardioverter-defibrillator prevents sudden death in patients with Chagas cardiomyopathy in the Brazilian Amazon
There are few published records of the occurrence of chagasic cardiomyopathy in the Amazon region, in which, ventricular aneurysms and ventricular tachyarrthythmias have been described; both of these are life-threatening clinical manifestations
29/01/2021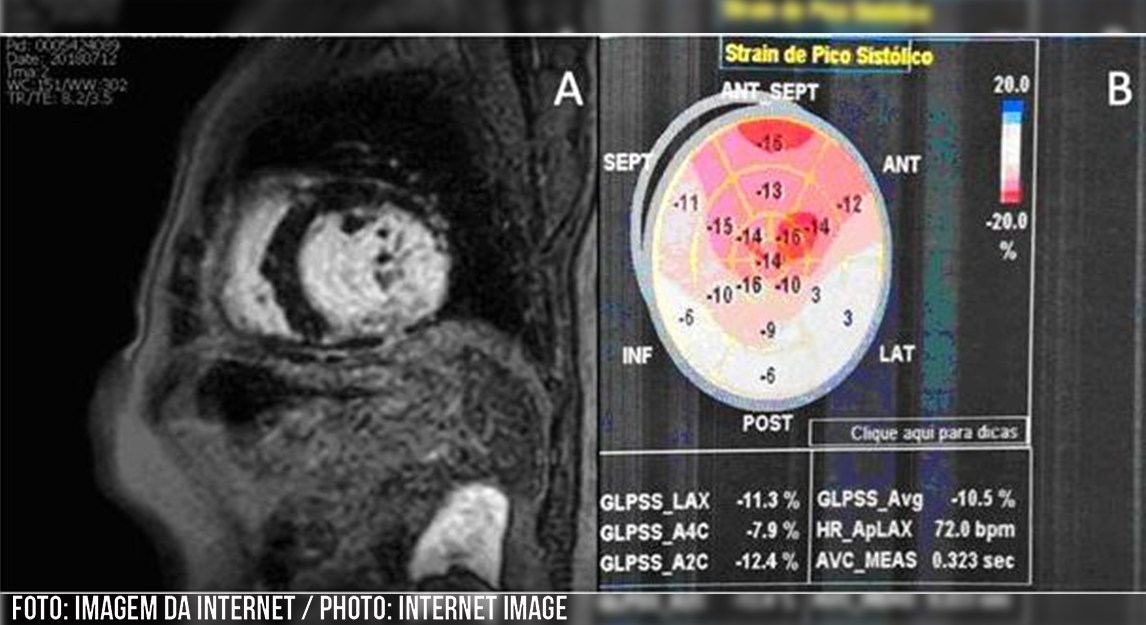
The article has been submitted and accepted by the Revista da Sociedade Brasileira de Medicina Tropical (Brazilian Journal of Tropical Medicine), where it will be fully published
Many patients with chronic Chagas disease cardiomyopathy do not know their dangerous disease and are at high risk of sudden death. Here, the Newsletter of the BSTM shows the life-saving benefits of an implantable cardioverter-defibrillator in reversing fatal ventricular tachycardia. The description is critical in the Amazon Region scenario, where chronic Chagas disease rare is unusual, despite being orally transmitted, and causing epidemics with high mortality. The patient was successfully followed and treated by a team from Manaus, Brazil, led by the cardiologist Dr. Kátia Couceiro.
The article has been submitted and accepted by the Revista da Sociedade Brasileira de Medicina Tropical (Brazilian Journal of Tropical Medicine), where it will be fully published.
Authors:
Katia N Couceiro, Jessica V Ortiz, Mônica R H S Silva, Débora R T Sousa, Kenny RSouza, Gabriela M Alencar, Laylah K C Magalhães, Maria das Graças V B Guerra, João M B B Ferreira and Jorge A O Guerra.
Institutions:
State University of Amazon, Tropical Medicine Foundation “Dr. Heitor Vieira Dourado” and Hematology and Hemotherapy Foundation of Amazon, Brazil.
Introduction to Chagas disease in Amazonia
Chagas disease (CD) is endemic in Latin America and has become a worldwide public health problem due to increased human migrations. It was considered a neglected disease in 2005. One hundred and ten years after its discovery, the World Health Organization now estimates that 70 million people are at risk of acquiring the sickness disease, seven million people are infected, and the disease annually1 causes 10,000 deaths (1).
This disease can manifest in two stages: an acute, symptomatic or asymptomatic form, and a chronic phase with indeterminate, cardiac, or digestive presentations. Approximately 60% of infected individuals have an indeterminate state, 25% to 35% develop heart disease, and of these, 10% may develop severe heart disease (2). In endemic regions, it is an important cause of sudden death. Chagas cardiomyopathy (CCM) manifests with arrhythmias, conduction disorders, heart failure, thromboembolic accidents, and premature death. Its pathogenesis is multifactorial and complex. Chronic inflammation can result in fibrosis and consequent sinus node dysfunction, atrioventricular and intraventricular conduction abnormalities, and ventricular tachyarrhythmias (2, 3).
The Amazon Region has long been considered a non-endemic area for CD; however, in recent decades, with an increase in the number of acute and chronic cases, this profile has changed. In the State of Amazonas, Brazil, the first patient with serologically positive chronic disease was recorded in 1973. Since then, surveillance programs and serological surveys have been carried out, and new cases have been detected. Previous studies suggest lower morbidity levels related to CD in the Amazon Region, probably due to different T. cruzi strains from those found in other endemic areas (3,4).
Despite this, a few cases of CCM have been reported in the Amazon. The first reports of dilated cardiomyopathy with chagasic etiology in this region date back to 2003, with two fatal cases (5), three autochthonous cases diagnosed in 2009 (6), and cases of ventricular tachycardia in 2012 (7).
In this study, the authors present a case report of successful cardioverter-defibrillator implantation to prevent sudden death in a patient with autochthonous CCM in the Brazilian Amazon.
Case Report
A 60-year-old man, a painter, currently living in the state capital, Manaus but born in Autazes in the interior of Amazonas, where he lived until the age of 18, was diagnosed with Chagas disease in 2015, after an attempt to donate blood. He developed the cardiac form of the disease and presented with dilated cardiomyopathy with ventricular dysfunction. His left ventricular ejection fraction (LVEF) was 44% using the Simpson method. An apical aneurysm was observed, and a baseline 12-lead electrocardiogram (ECG) showed an abnormal sinus rhythm and changes in ventricular repolarization in the lower-lateral wall. On the 24-hour Holter recording, he presented 16,881 isolated, bigeminal, polymorphic ventricular arrhythmias.
In 2018, cardiac magnetic resonance imaging was performed, revealing increased cavity volumes and significant left ventricular dysfunction in addition to a late transmural enhancement in the inferior–medial–basal wall (Figure 1A). Furthermore, an echocardiogram showed a global longitudinal strain of -10.5% and a considerably low LVEF of 29% (Figure 1B). A positive high-resolution ECG for detecting late potentials was performed, and 12% myocardial fibrosis was identified using the Selvester score (8).
In August 2019, he was admitted to the emergency department with tachycardic palpitations, cold sweats, and syncope. Monomorphic ventricular tachycardia without a pulse was monitored and recorded on the ECG (Figure 2A), which led to a cardiopulmonary arrest at the emergency room. He was immediately subjected to cardiac resuscitation maneuvers and was reverted to tachyarrhythmia after a 200-J biphasic shock considering he presented with isolated ventricular tachycardia.
Since the diagnosis of CD and CCM, the patient has been on medication optimized for heart failure: enalapril 20mg/day, carvedilol 50mg/day, and spironolactone 25mg/day. During the cardiorespiratory arrest episode with pulseless monomorphic ventricular tachycardia and after its reversal with defibrillation, the patient was referred for an implantable cardioverter-defibrillator (ICD) as secondary prophylaxis for sudden death.
After discharge from the hospital, he was referred for outpatient follow-up. He was prescribed medication for heart failure combined with 400 mg/day amiodarone to avoid arrhythmic storms. Twenty-four-hour Holter monitoring was performed again, which demonstrated the absence of ventricular arrhythmias. Approximately three months after ICD implantation, the patient presented with an ICD shock episode, documented by intracavitary electrogram during telemetric evaluation (Figure 2B and 2C), automatically reverted by the implanted cardioverter-defibrillator.
Discussion
There are few published records of the occurrence of chagasic cardiomyopathy in the Amazon region, in which ventricular aneurysms (6) and ventricular tachyarrthythmias (7) have been described; both of these are life-threatening clinical manifestations (9).
Marques et al. (2012) (7) reported the first known case of ventricular tachycardia in an autochthonous patient in the Amazon. This report now highlights the importance of the continuous monitoring of patients with CD in Amazonia. Monitoring allows the identification of predictors of arrhythmias, and the lower the severity and permanent damage, the better the prognosis. Consequently, the lower the cost burden on the public health system is, the better the patients social and economic quality of life will be (10).
Although both early diagnosis and monitoring are essential, the patient in this report already had myocardial fibrosis identified by cardiac magnetic resonance, with late myocardial enhancement. Malignant ventricular arrhythmias are more prevalent than other forms of heart disease in patients with CD. As sudden cardiac death is commonly observed in CD, this cardiomyopathy is a frequent indication for ICD implantation (10).
Myocardial fibrosis is identified using cardiac magnetic resonance. Late myocardial enhancement serves as a meaningful predictor of arrhythmias and sudden death in several ischemic and non-ischemic cardiomyopathies, such as hypertrophic cardiomyopathy and CD. It should be considered for patient monitoring and assessment of severity and interventions (11).
The arrhythmogenic form of CD is a significant cause of mortality (2). Although it has been infrequently reported in the Amazon Region, the arrhythmogenic form of CCM is an important cause of sudden death in the area, as evidenced in our case.
In recent decades, the occurrences of CDs acute and chronic cases have increased significantly in regions where diagnostic and therapeutic resources are scarce. This case report reinforces the need to improve regional diagnostic resources, identify patients at the most significant risk, and prevent sudden death.
References
- World Health Organization (WHO). Fourth WHO Report on Neglected Tropical Diseases: Integrating Neglected Tropical Diseases into Global Health and Development. Geneva: WHO; 2017, 271 p.
- Marin-Neto JA, Cunha-Neto E, Maciel BC, Simões MV. Pathogenesis of chronic Chagas heart disease. Circulation. 2007;115(9):1109–23.
- Brum-Soares LM, Xavier SS, Sousa AS, Borges-Pereira J, Ferreira JMBB, Costa IR, et al. Morbidade da doença de Chagas em pacientes autóctones da microrregião do Rio Negro, Estado do Amazonas. Rev Soc Bras Med Trop. 2010;43(2):170–7.
- Coura JR, Junqueira AC, Ferreira JMB. Surveillance of seroepidemiology and morbidity of Chagas disease in the Negro River, Brazilian Amazon. Mem Inst Oswaldo Cruz. 2018;113(1):17–23.
- Albajar PV, Laredo SV, Terrazas MB, Coura JR. Miocardiopatia dilatada em pacientes com infecção chagásica crônica. Relato de dois casos fatais autóctones do Rio Negro, Estado do Amazonas. Rev Soc Bras Med Trop. 2003;36(3):401–7.
- Ferreira JMBB, Guerra JAO, Barbosa MGV. Ventricular aneurysm is a chronic chagasic patient from the Brazilian Amazon. Rev Soc Bras Med Trop. 2009;42(4):474-5.
- Marques AEAS, Ferreira JMB, Maldonado JGA, Santos FGC, Costa DA, Resende GAS, et al. Morte súbita abortada em paciente chagásico crônico na Amazônia Brasileira: relato de caso. Rev Hosp Univ Getúlio Vargas. 2012;11(1):44–8.
- Loring Z, Chelliah S, Selvester RH, Wagner G, Strauss DG. A detailed guide for quantification of myocardial scar with the Selvester QRS score in the presence of electrocardiogram confounders. J Electrocardiol. 2011;44(5):544–54.
- Sarabanda AVL, Marin-Neto JA. Predictors of mortality in patients with Chagas cardiomyopathy and ventricular tachycardia not treated with implantable cardioverter-defibrillators. PACE – Pacing Clin Electrophysiol. 2011;34(1):54–62.
- Barbosa MPT, Da Costa Rocha MO, De Oliveira AB, Lombardi F, Ribeiro ALP. Efficacy and safety of implantable cardioverter-defibrillators in patients with Chagas disease. Europace. 2013;15(7):957–62.
- Senra T, Ianni BM, Costa ACP, Mady C, Martinelli-Filho M, Kalil-Filho R, et al. Long-Term Prognostic Value of Myocardial Fibrosis in Patients With Chagas Cardiomyopathy. J Am Coll Cardiol. 2018;72(21):2577–87.
FIGURE 1: A) Cardiac magnetic resonance imaging with the presence of late transmural enhancement in the lower-middle-basal wall; B) Transthoracic echocardiogram with global longitudinal strain (-10.5%)
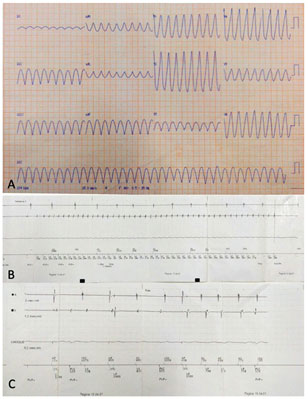
FIGURE 2: A) Baseline ECG with monomorphic ventricular tachycardia without a pulse; B) Sustained ventricular tachycardia detected by the intracavitary electrogram; C) Intracavitary electrogram stimulating the atria and ventricles after ICD shock










