Study proves the effectiveness of medicine to treat Chagas
Brazilian researchers have found that the antiparasitic drug Benznidazole can improve the prognosis of patients with Chagas disease in the chronic phase
13/12/2018
This is one of the most comprehensive studies of its kind demonstrating a clinical benefit of Benznidazole
An important study on Chagas disease, conducted by a group of Brazilian researchers, whose first author is Professor at the Federal University of São João Del-Rei Medical School (UFSJ/Divinópolis, Minas Gerais), Dr. Clareci Cardoso, investigated the effects of the drug Benznidazole, distributed free of charge by the Unified Health System (SUS) to carriers of Chagas disease. The research was recently published in the journal PLOS Neglected Tropical Diseases.
To Dr. Clareci, who holds postdoctoral degrees in Epidemiology at University of California Berkeley, USA, and in Tropical Medicine at São Paulo University (USP), the scientific knowledge produced by the project reaffirms the importance of partnerships between research groups and institutions to strengthen Brazilian science. “We believe that the results of our research can have a positive impact on society, contributing to the reduction of morbidity and mortality associated to diseases that are still neglected.
The results of our investigation suggest that treatment with benznidazole should be carried as soon as possible, before the patient with Chagas disease develops advanced heart disease, that is, before 50 years of age, said Dr. Clareci Cardoso, with whom the press advisory of the Brazilian Society of Tropical Medicine (SBMT) spoke.
Check the full interview:
BSTM: according to research in which you are one of the authors, if used in the chronic phase (initial) of disease, Benznidazole treatment can improve the clinical and parasitological results in patients with Chagas. Is there any study about this drug being used during the acute phase of the disease?
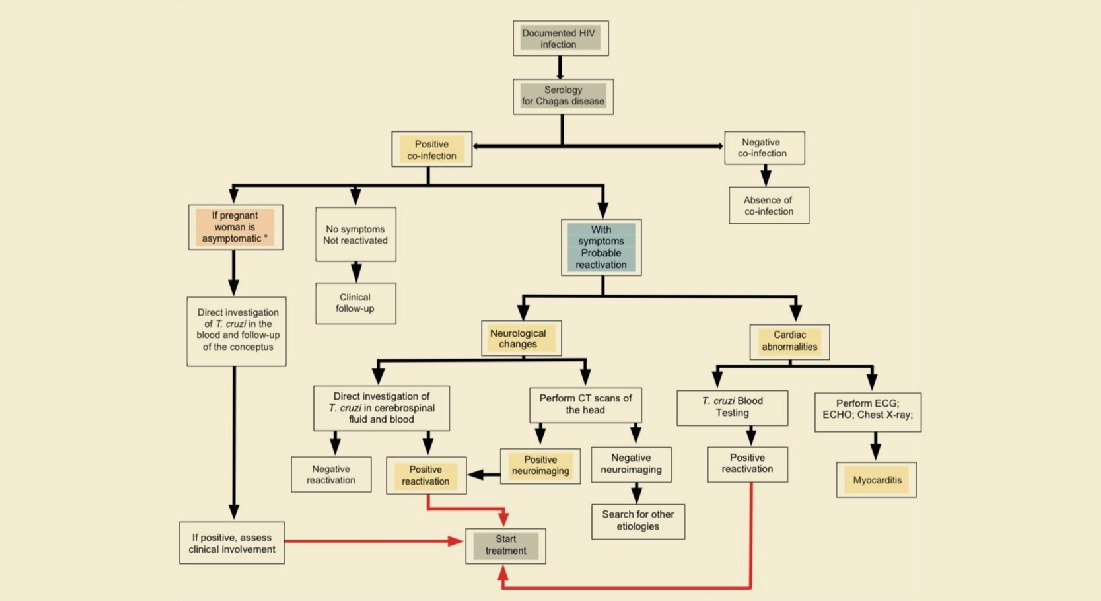
Dr. Clareci Cardoso: Clinically, Chagas disease is divided into acute and chronic phases. The acute phase resolves itself spontaneously within 2 to 4 months. Without any treatment, it becomes chronic and persists throughout the individuals life. In the chronic phase, the main complication is chagasic cardiomyopathy. The majority of scientific studies featuring benznidazole effectiveness are focused on the acute phase of Chagas disease. A great part of the patients in the initial phase have success in the treatment, especially children, who have a higher tolerance to the medications when compared to adults. On the other hand, during the chronic phase the results of effectiveness are controversial in literature, either due to methodological differences in the studies, patients’ reinfection in endemic areas or by the diversity of circulating parasite species.
The clinical guidelines for Chagas disease (Ministry of Health 2018) recommend that patients in the acute phase should engage in the treatment immediately. Although drugs have similar efficacy, benznidazole should be the first option to be prescribed, due to greater usage experience in our environment, higher availability, including pediatric presentations, and the profile of adverse events. Nifurtimox may be used in cases where benznidazole is not adequately tolerated.
Several studies show that both antiparasitic medications are effective in reducing the severity of symptoms and the duration of detectable parasitemia in Chagas disease, both in the acute phase as in the congenital form of the disease. It is believed that cure occurs in 60% to 85% of the patients in the acute phase and, in over 90% of newborns with congenital infection in the first year of life.
BSTM: The benznidazole provided by the Unified Health System (SUS) has always raised questions in the medical field regarding its efficacy. What are the reasons or why these doubts exist?
Dr. Clareci Cardoso: There isnt a cure biomarker for Chagas disease. The diseases evolution is long lasting, and for this reason, large clinical trials have not been held, demonstrating that the drug changes its clinical evolution in patients in an indefinite way. The only study is recent (BENEFIT study) and it assessed people with established cardiomyopathy. In this group, the disease showed no evolution improvement. Physicians are insecure to offer a drug with many side effects without knowing exactly how effective it is. Our recently published research is an observational study; however, the statistical analysis simulates a clinical trial, and therefore, brings numbers that can aid in the treatment decision.
In Brazil, Benznidazole is delivered free of cost to patients by the SUS. As mentioned in the previous question, it is more effective if prescribed during the initial phase of the disease, i.e., close to parasite contamination. However, in Brazil, many Chagas disease diagnosis are not held in an opportune manner, considering that many patients live in remote areas and very far away from diagnostic resources; this way, treatment takes place in the later stage, many times when the patient already has established the heart disease. In these cases, it presents a reduced effectiveness.
BSTM: Carlos Chagas announced the detection of the Chagas disease causative agent, the protozoan Trypanosoma cruzi in humans in 1909. Even having spent almost 110 years since the announcement, why is Benznidazole still the only drug used to treat Chagas disease in Brazil?
Dr. Clareci Cardoso: In general, what is observed is very little interest of pharmaceutical industries in funding this field, which is itself, considered to be neglected. Chagas disease poses another problem: clinical trials are difficult because their outcomes take a long time to appear, and there is no established cure biomarker. Even to develop a drug, its hard to prove that it really works. Clinical trials currently use the PCR as an outcome. Since PCR can be very negative in many patients who develop cardiomyopathy, some researchers disagree this could be an effective biomarker to indicate the cure of the disease.
BSTM: In the USA, the Food and Drug Administration only approved the registration of the drug Benznidazole, made by Chemo Group, on August 29, 2017. It is the first medication licensed by the FDA to treat Chagas disease. In your opinion, why did they take so long to approve it?
Dr. Clareci Cardoso: Chagas disease was not seen as a problem, and as mentioned previously, efficacy and effectiveness studies of BZN are difficult. Another fact to consider is that effectiveness trials in the acute phase are old and did not meet the very strict rules established by the FDA.
BSTM: Is it correct to say that the approval of Benznidazole was a major milestone in the United States, as well as a global response to Chagas disease? Why?
Dr. Clareci Cardoso: Absolutely. The approval of the BZN shows that Chagas disease is now seen as a problem in the USA. It is no longer a problem restricted to some countries and became a worldwide concern, considering the migration of patients into or out of endemic areas. This has changed the diseases epidemiology and patients diagnosed with Chagas disease have become increasingly more frequent. Another factor that contributed to the prevalence increase is the deployment of Chagas disease screening test in American blood banks. This way, a prevalence increase, by broadening diagnostic access, makes it necessary to provide treatment for this population.
BSTM: Benznidazole produces heavy side effects such as nausea, headaches and vomiting. In your opinion, does this make patients abort treatment?
Dr. Clareci Cardoso: Yes, Absolutely. These adverse reactions are common, and so the administration of benznidazole needs to be properly overseen by periodic visits to the medical professional, and if necessary, to have doses adjusted or added the use of alternative medicine (or benznidazole nifurtimox).
BSTM: Researchers recently used quinidine, prescribed in the standard guidelines against malaria, to treat Chagas disease. In tests, the medicine prevented the kissing bug from transmitting the Trypanosoma cruzi. Do you believe this could become an option, with less side effects, for patients with the disease? Why?
Dr. Clareci Cardoso: Fortunately, we have had other alternatives to control Chagas disease, in this sense, knowledge produced in universities is fundamental, considering this area lacks investments by pharmaceutical companies. As for this study using the quinidine, the results were promising for vector control, however, we have to follow other investigations with caution to evaluate its effectiveness and efficacy in animal and human models.
If the substance is proved to be effective controlling Chagas disease in humans, it will be fantastic, because we know that both available medications, benznidazole and nifurtimox, result in severe adverse reactions, many times making the patients abort treatment and thus, the irreversible damages our patients suffer from the parasites persistence in the organism.
BSTM: The finding of your research group came at a rather troubled time in the national economy, where the government has imposed cutbacks in resources research funds, hampering the advances of science and the production of new discoveries. Is it correct to affirm that the findings strengthen Brazilian science, posing against the neglect with public universities and the constant struggle for research investment, and thus, becoming more important and resolute?
Dr. Clareci Cardoso: We think this is an important moment for Brazilian science to present innovative results. In Brazil, we have researchers with internationally renowned academic backgrounds and important public education institutions. We know that investments for research has not met all of our demands, but the institutional partnerships have favored scientific production. An example of success has been the São Paulo & Minas Gerais Research Group in Tropical Diseases (SaMi_Trop), where researchers from USP, UFMG and UFSJ/Divinópolis and UNIMONTES develop a large research project in Chagas disease, with the follow-up of 2,157 patients in Northern Minas Gerais and Vale do Jequitinhonha, aiming to monitor the evolution of the disease and to identify progression biomarkers. This project is funded by the National Institute of Health (NIH), USA, and some other institutions, including FAPESP, FAPEMIG and CNPq. It also counts with the Blood Systems Research Institute and the University of California (USA).
The scientific knowledge produced within this project reaffirms the importance of the partnership between groups and institutions to strengthen Brazilian science. We believe that the results of our research can have a positive impact on society, contributing to the reduction of morbidity and mortality associated to diseases that are still neglected.
BSTM: What are the next steps of the research?
Dr. Clareci Cardoso: The results of our investigation suggest that treatment with Benznidazole should start as soon as possible, before the patient with Chagas disease causative develops advanced heart diseases, i.e., before the 50 years of age. Patients previously treated with benznidazole showed significant reduction of parasitemia, lower frequency of severe cardiomyopathy markers and reduced mortality. If used in the early stages of Chagas disease, treatment with benznidazole may result in better clinical and parasitological indicators.
The most important contribution of the present study is the demonstration of a significant clinical benefit of benznidazole, which includes reduction in well-established markers of disease severity such as, typical Chagas abnormalities in ECG, high levels of NT-proBNP or both, as well as lower mortality during the two-year follow-up of patients.
Since these patients are part of a prospective cohort, they will be monitored over time for evaluation of other outcomes such as disease progression over the years, impact of the disease in life quality and others, as well as observing the maintenance of the benznidazole positive impact for a greater period of observation.…