
WHO issues warning regarding falsified meglumine antimonials vials in Iran and Pakistan
Organização Mundial da Saúde pede maior vigilância para esses produtos específicos. O fato é muito grave e pode acontecer no Brasil
05/06/2019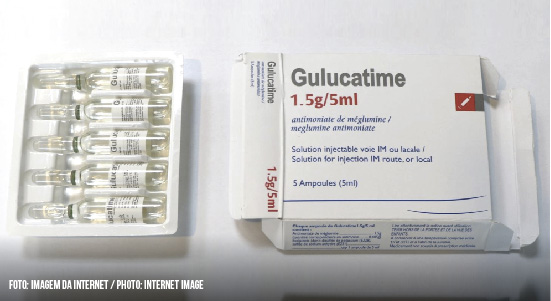
To date, the WHO has not been notified of adverse reactions attributed to the use of one of these two counterfeit products
In January, the World Health Organization (WHO) was informed that in Iran patients could buy a product called GULUCATIME, purportedly manufactured by Tillotts Pharma AG, however, checks by WHO confirmed that the product was counterfeit. The product is presented in packs containing five ampoules and the package, labeled in English and French, contains spelling errors in both languages.
In March, the WHO was informed that in Pakistan patients could purchase a similar product called GLUCATIME, allegedly also manufactured by Tillotts Pharma AG, whose falsification was also confirmed. The results of the laboratory analysis provided by WHO indicated that the product was not manufactured in accordance with good manufacturing practices.
Although the packaging or leaflets of both products (Gulucatime and Glucantime) indicate that they are manufactured by Tillotts Pharma AG, the company has confirmed to the WHO that it does not manufacture, subcontract, manufacture or distribute these products anywhere in the world.

Dr. Ali Khamesipour, from Tehran, says that this type of alert is common in Iran, but this event is rare for leishmaniasis. The professor explains that the medications used for the treatment of the disease are imported by the government (Ministry of Health) and distributed in the public health network for patients and that, in this case, the ampoules of meglumine antimoniate were purchased by a company and sold to the Ministry of Health, as the ampoules and packaging were similar to the original Glucantime, the authorities did not suspect that they were fake. For him, this counterfeiting could have been avoided and hopes that it will not happen again.
To avoid such occurrences, the WHO calls for greater vigilance in the supply chains of countries that may be affected by these counterfeit medical products. Such expanded surveillance should include hospitals, clinics, health centers, wholesalers, distributors, pharmacies and any other medical product providers.
Dr. Khamesipour warns that patients who have used the counterfeit medicine should stop using the medication. Since the results of the analysis are not yet ready, we do not know how to prevent toxicity and side effects. Health professionals are critical in this situation and can help by contacting patients and passing on the information and should also collect any unused medicine, he adds.
Cases of counterfeiting have occurred in Iran, Afghanistan, Uzbekistan and Pakistan. In the case of Iran, the fact occurred due to the lack of medication. According to Professor Khamesipour there was no commercialization. The Ministry of Health acquired 50 thousand ampoules at an original cost of USD 1,20 each. This was the first purchase order that was to be repeated on a larger scale, but was fortunately interrupted, he says.
Even for health professionals who deal with this type of treatment and make applications regularly it is virtually impossible to differentiate a counterfeit product from a good one. It is not easy to distinguish between original and counterfeit drugs. Health professionals should be very cautious, and should they notice any changes in medication or adverse effects in patients, they should inform the authorities immediately and take all care to stop the use of the medicines, adds Dr. Khamesipour. Finally, he emphasizes that authorities should always purchase medications from the original sources and consult specialists before buying drugs.
The WHO has a page that aims to ensure a timely, accurate and consistent response to health events arising from medical products that pose a threat international public health. Read the Medical Product Alert Nº 7/2019 here.…










